Blog
Quality Lab Proficiency Testing – Once a Best Practice; Now a Standard Requirement

The FDA embarked on a new endeavor, “Smarter Food Safety,” that is becoming a reality. It all started with a vision, and the blueprint has now been published and has begun to take root.
These changes are wide-sweeping and add substantial value to public health. Food companies should educate themselves and prepare as the blueprint takes shape and becomes standard. Where to start, how to prepare, and how companies will be impacted are common topics reverberating on social media.
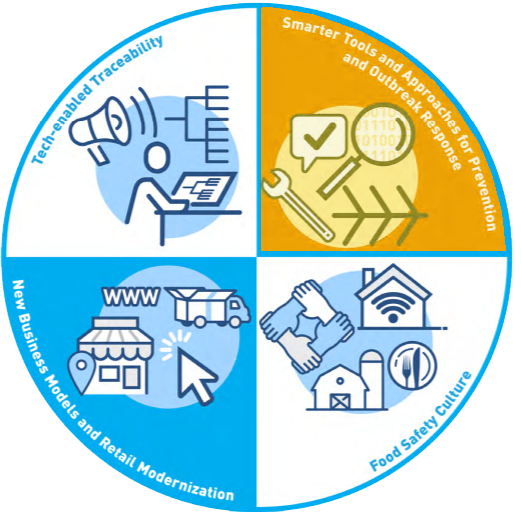
Core Element 1: Tech-Enabled Traceability is the backbone of the entire initiative and is the driving force that propels Core Element 2: Smarter Tools and Approaches for Prevention and Outbreak Response.
The philosophy is simple: create a system of networked sensors, machines, or analyzers that all feed into a database instantaneously to manage outbreaks, physical adulterants, and possible contaminants or dangers to public health. The FDA intends implementation to be scalable to companies of all sizes, from ten to ten thousand employees, and not be overly burdensome. This network should be along the entire supply chain from raw to retail and create a complete story of where the product came from, how it was processed and transported, and where it was sold. Along the path, the entire process will be traceable in the event of a recall and specific error points captured for corrective action.
Where to Begin?
It all starts in receiving. Whether it’s raw grain, raw ingredients, or unpasteurized milk fresh from the dairy, the raw product is sampled, analyzed, and logged. This process is already standard practice, but the data integration into the company network may be a loose string in current manufacturing. Are you using a system that provides the date, time, result of analysis, and release? Or are you printing out a ticket or writing it down manually? How are you logging your environmental monitoring program, chemical hazard control checks, or pathogen control plan throughout manufacturing? In most places, it is a manual process to some extent. Swabs are taken, plated, read manually, and then manually reported into Laboratory Information Management System (LIMS).

Confectionery factory employees in white coats packing
Smarter Food Safety Solutions
A more integrated solution is needed to prepare for the eventuality that the FDA will require stricter compliance to the New Era of Smarter Food Safety Blueprint. There are several systems available, and for Charm, it is the Charm SMART or Charm CONNECT data management systems.
Imagine a world where a tanker or hauling truck enters receiving and the raw product is tested and cleared. The test data with manifest, date, time, location, and results are automatically sent to the LIMS system. As the Quality Team takes environmental and ATP swabs, the results sync directly to that specific product. When the final product testing is complete, the results are all packaged together with lot-to-lot distinction. And…there is no change in your current Standard Operating Procedures (SOP’s) and processes. So there is very little extra work on your end. All you are doing is securing proof of your hard work.
In preparation for Smarter Food Safety Blueprint becoming a compliance guideline, Charm Sciences created a networked data management system that directly ties our Antibiotic, Mycotoxin, microbial detection, and ATP monitoring systems into your existing LIMS system. It can work within a single facility or across an entire corporation with multiple facilities. This approach to data management makes FDA audits and inspections approachable and seamless. Not only is the data compiled, but the files and reports that encompass all aspects of your Quality Management System (QMS) directly address FDA Core Values 1 and 2. Further, automated recording removes an enormous amount of human error with the added benefit of data security for the entire system.
Conclusion
The FDA’s vision can add substantial value to your facility or organization’s QMS. In the event of an issue, you have instant results to correct that issue, and if the product makes it out the door, you have a complete trail that drives your corrective actions. In the event of a recall, you can use the information to prove that you were not the weak link in the chain and the issue did not derive from your facility.
We are available to help prepare you for the New Era of Smarter Food Safety.
For more information, please feel free to contact us at any time.
About Charm Sciences
Established in 1978 in Greater Boston, Charm Sciences helps protect consumers, manufacturers, and global brands from a variety of issues through the development of food safety, water quality, and environmental diagnostics tests and equipment. Selling directly and through its network of distributors, Charm’s products serve the dairy, feed and grain, food and beverage, water, healthcare, environmental, and industrial markets in more than 100 countries around the globe.
